Reassembly
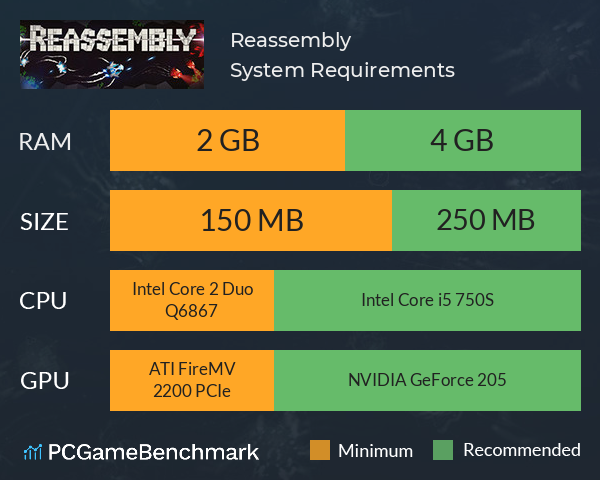
Steamworld dig 2 free download. Reassembly is an Indie video game developed by Anisoptera Games ( Arthur Danskin ) with the aid of Indie Voyage on Kickstarter. The game is fully released on Steam, and has a deep and thriving community built up around community managed Tournaments, and modifications.
In this work, we propose Content-Aware ReAssembly of FEatures (CARAFE), a universal, lightweight and highly effective operator to fulfill this. Reassembly Instructions. Follow the steps below to reassemble the game components after playing Mystery at the Stargazer's Manor. Pass the game on to a.
The game was fully released on February 2015, but thanks to the commitment of the game's developer, new features continue to be added. ABOUT THIS WIKI This wiki was originally founded by alpha testers to build up a wikia database about the game. We strive to let this wiki be known to the general public with great contributors and editors, and eventually build up a community on Wikia along with the Reassembly Forums, our place of origin, and the new and rapidly growing Discord group. If you are new to wikis and wish to help this wiki develop, please feel free to check the.
Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 (both PDF and HTML) across all institutions and individuals. These metrics are regularly updated to reflect usage leading up to the last few days.Citations are the number of other articles citing this article, calculated by Crossref and updated daily.The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric.com with additional details about the score and the social media presence for the given article. Find more information on. Zr/Hf aqueous-acid clusters are relevant to inorganic nanolithography, metal–organic frameworks (MOFs), catalysis, and nuclear fuel reprocessing, but only two topologies have been identified. The ( Zr 4) polyoxocation is the ubiquitous square aqueous Zr/Hf-oxysalt of all halides (except fluoride), and prior-debated for perchlorate.
Simply adding peroxide to a Zr oxyperchlorate solution leads to a striking modification of Zr 4, yielding two structures identified by single-crystal X-ray diffraction. Zr 25, isolated from a reaction solution of 1:1 peroxide/Zr, is fully formulated Zr 25O 10(OH) 50(O 2) 5(H 2O) 40(ClO 4) 10 xH 2O. Zr 25 is a pentagonal assembly of 25 Zr-oxy/peroxo/hydroxyl polyhedra and is the largest Zr/Hf cluster topology identified to date. Yet it is completely soluble in common organic solvents. ZrT d, an oxo-centered tetrahedron fully formulated Zr 4(OH) 4(μ-O 2) 2(μ 4-O)(H 2O) 12(ClO 4) 6 xH 2O, is isolated from a 10:1 peroxide/Zr reaction solution. The formation pathways of ZrT d and Zr 25 in water were described by small-angle X-ray scattering (SAXS), pair distribution function (PDF), and electrospray ionization mass spectrometry (ESI-MS). Zr 4 undergoes disassembly by 1 equiv of peroxide (per Zr) to yield small oligomers of Zr 25 that assemble predominantly in the solid state, an unusual crystal growth mechanism.
The self-buffering acidity of the Zr-center prevents Zr 25 from remaining intact in water. Identical species distribution and cluster fragments are observed in the assembly of Zr 25 and upon redissolution of Zr 25.
On the other hand, the 10:1 peroxide/Zr ratio of the ZrT d reaction solution yields larger prenucleation clusters before undergoing peroxide-promote disassembly into smaller fragments. Neither these larger cluster intermediates of ZrT d nor the smaller intermediates of Zr 25 have yet been isolated and structurally characterized, and they represent an opportunity to expand this new class of group IV polycations, obtained by peroxide reactivity and ligation. Terms & ConditionsElectronic Supporting Information files are available without a subscription to ACS Web Editions.The American Chemical Society holds a copyright ownership interest in any copyrightable SupportingInformation. Files available from the ACS website may be downloaded for personal use only. Users arenot otherwise permitted to reproduce, republish, redistribute, or sell any Supporting Informationfrom the ACS website, either in whole or in part, in either machine-readable form or any other formwithout permission from the American Chemical Society.

For permission to reproduce, republish andredistribute this material, requesters must process their own requests via the RightsLink permissionsystem. Information about how to use the RightsLink permission system can be found at.